Why IgE?
Potent immune effector function
- IgE has evolved to provide immunity to parasites which requires very potent immune effector function
- Mediated by high-affinity IgE receptor FcεRI on cells of myeloid lineage, including macrophages and monocytes
- Very tight binding of IgE by FcεRI receptor redirects tropism of myeloid cells and facilitates immunosurveillance
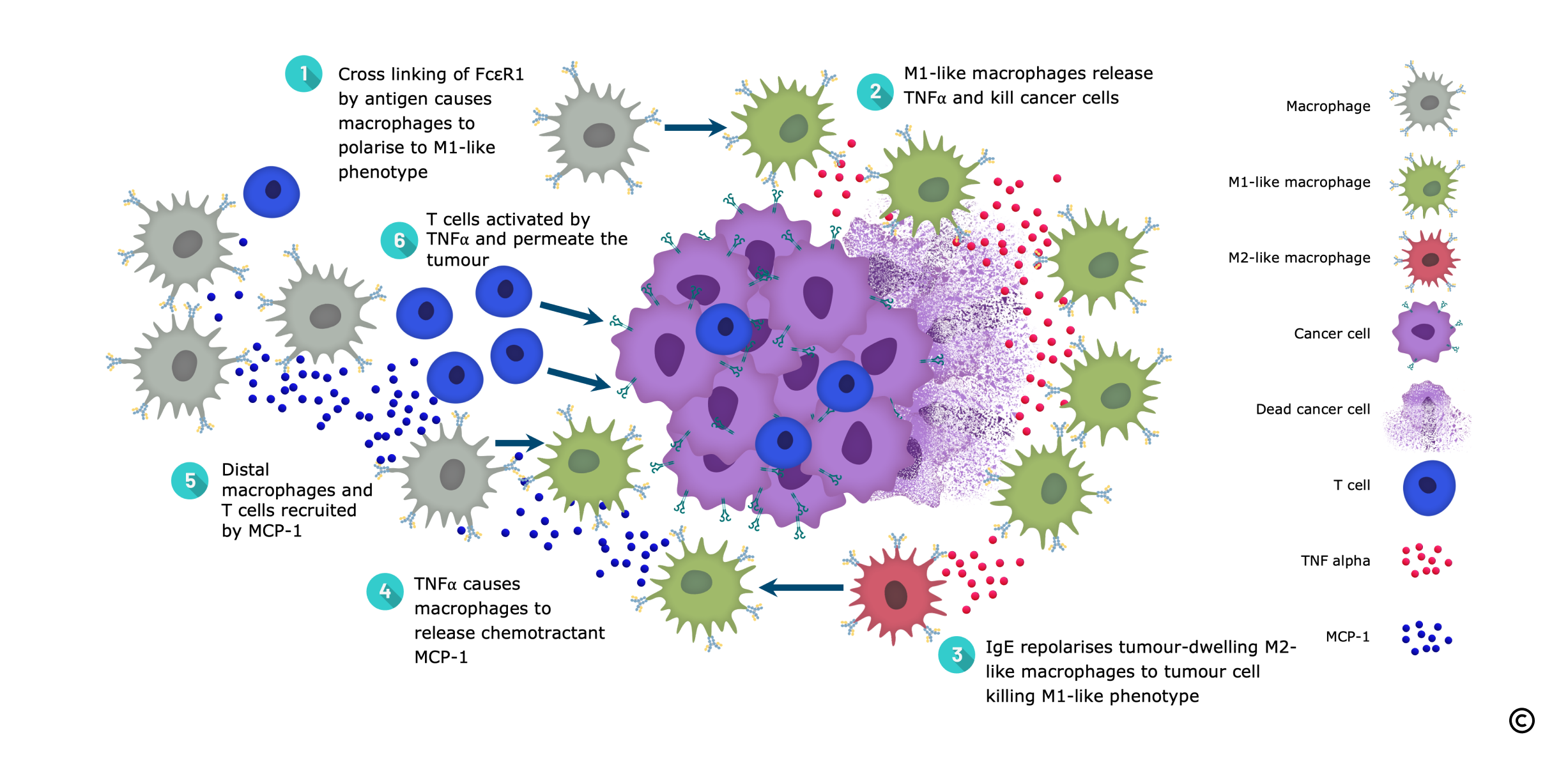
- IgE repolarises macrophages towards the anti-cancer, pro-inflammatory M1 phenotype
- IgE facilitates tumour permeation by macrophages
- IgE is able to kill tumour cells expressing very low levels of antigen, a strong point of differentiation
Unique Mechanism of Action facilitates combinations with other modalities
- IgE has demonstrated strong single agent activity and its unique MoA allows combination with other modalities such as checkpoint inhibitors and ADCs
Longer tissue half-life
- IgE has a long tissue half-life of 2 weeks versus 2–3 days for IgG
- The converse is true in serum where IgE has a half-life of 2–3 days versus 2–3 weeks for IgG
- IgE’s long tissue half-life is ideal for treating solid tumours representing 92% of all cancers
IgE has no inhibitory receptor and is present at very low levels in serum
- Therapeutic IgE would be expected to have greater potency and lower dose levels in man
- Phase I data backs this up with dose levels >100X lower than typical IgG doses
IgE is a Multi-faceted Anti-cancer Modality
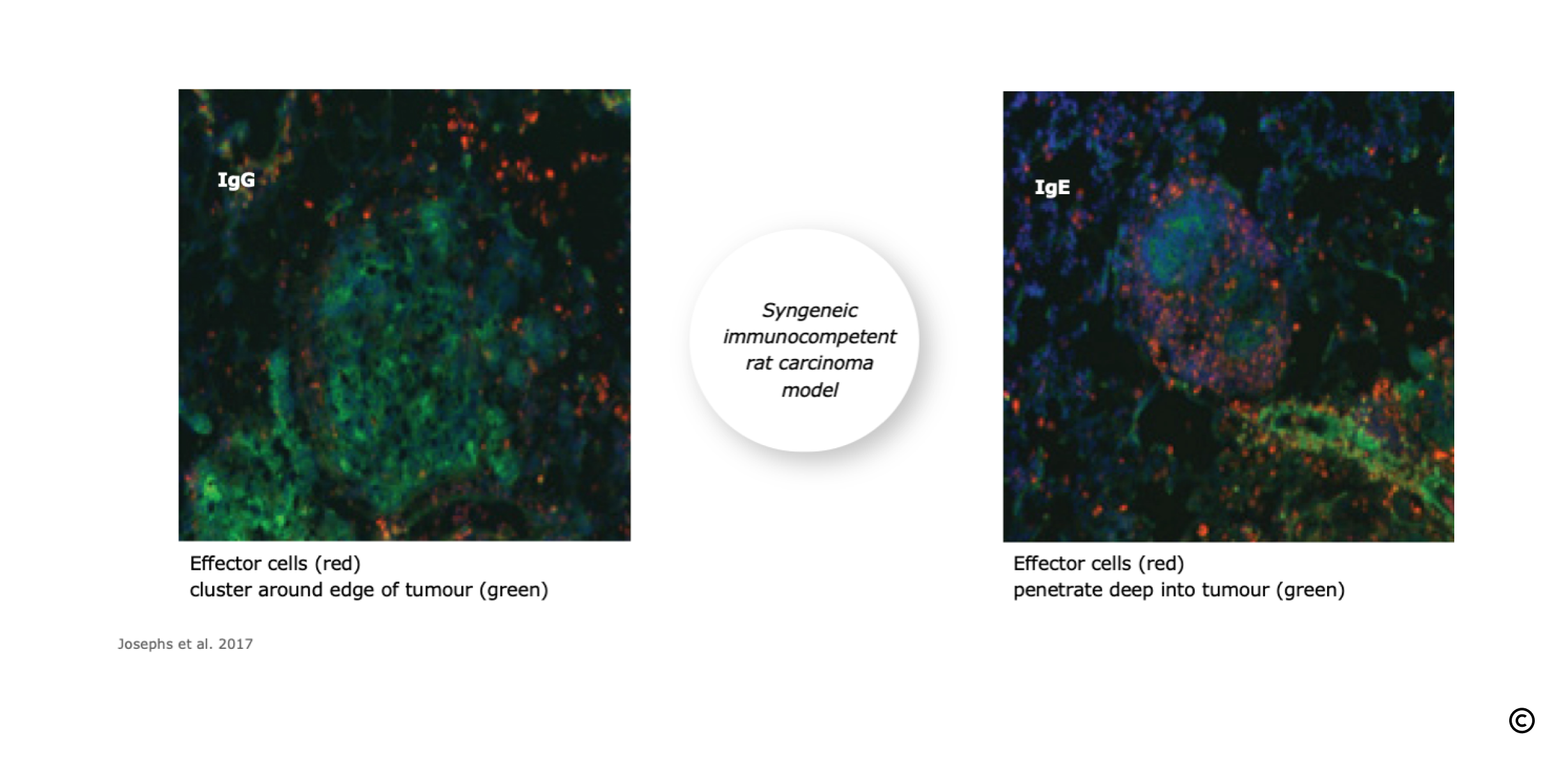
IgE Facilitates Effector Cell Permeation of Solid Tumours
Tumour permeation by macrophages is significantly increased with IgE versus IgG.
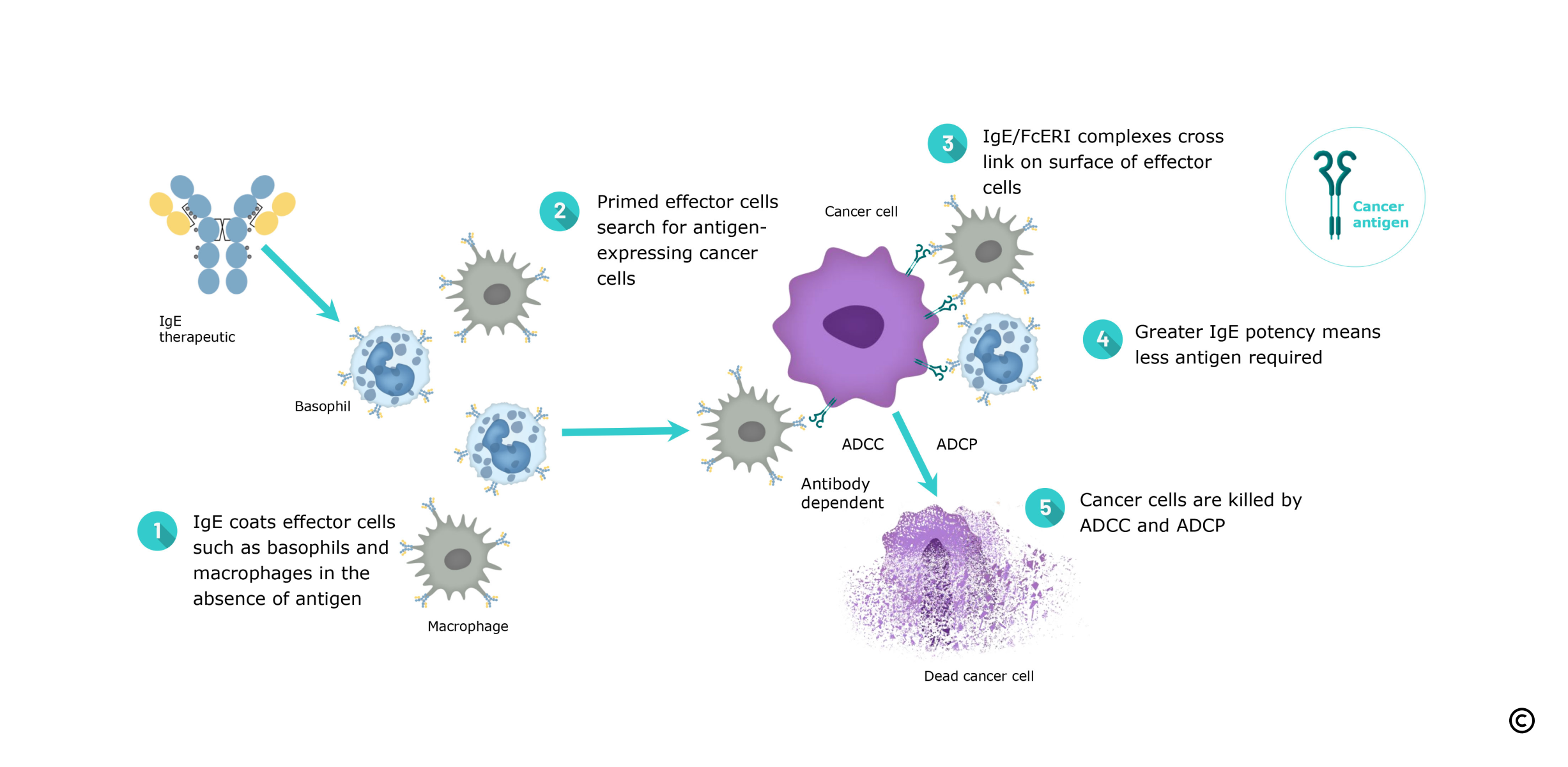
IgE Primes Immune Effector Cells for Cancer Cell Destruction
Immunoglobulin E (IgE) antibodies, well known for their role in allergic diseases and for contributions to antiparasitic immune responses, have several unique properties compared to existing cancer immunotherapies that make them well-suited to targeting solid tumours.
IgE Facilitates Tumour Destruction by Macrophages and T Cells